Making a beeline
Synopsis : Honey bees use a range of navigation skills including path integration – to shorten return flights – combined with map-like spatial memories to relocate the hive.
Introduction
Regular readers will be aware that I’m interested in the origins of words. The Oxford English Dictionary (OED) is a fantastic source of information and produces a free Word of the Day email {{1}}. This includes both the meaning and etymology of one word each day.
Since the complete dictionary includes over 600,000 words it will take a few years to collate the 20 volumes that comprise the entire dictionary {{2}}.
At the beginning of this week the word of the day was beeline.
The word beeline of course means:
A straight line or course, such as a bee follows in returning to its hive after having collected a full load of nectar; (occasionally) the course taken by a bee.
The word originated in the US almost 200 years ago. It was first recorded in the American Quarterly Review in June 1828. Anyone who has read Tom Seeley’s Following the Wild Bees will appreciate the context in which the word beeline was used:
The bee-hunter..encloses them [sc. bees] in a tube, and letting one fly, marks its course, by a pocket compass. Departing to some distance, at right angles to the bee-line just ascertained, he liberates another, observes its course, and thus determines the position of the hive, which lies in the angle made by the intersection of the bee-lines.
Beelining is the art of finding feral or wild colonies by following the returning flight of bees. The book had a companion website with some interesting videos if you’d like to know more.
Find and tell
Beelining ‘works’ because bees fly in a straight line back to the nest {{3}}.
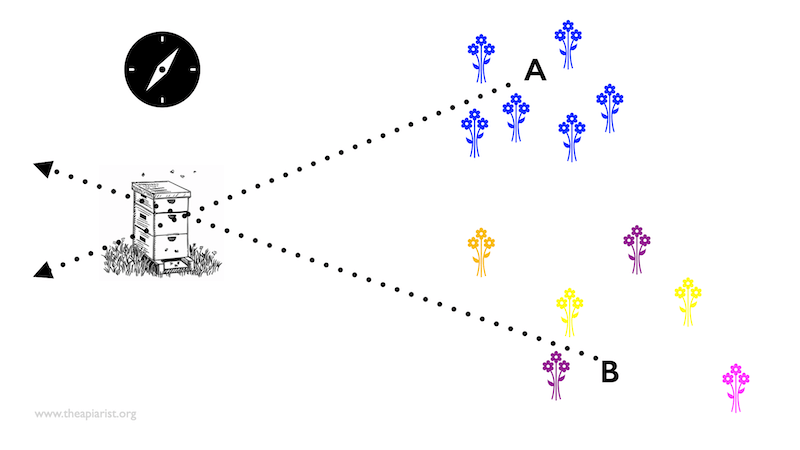
Assume the blue flowers above are nectar-rich and favoured by the bees. You capture a couple of bees feeding on the blue flowers and give them some additional syrup so that they are replete and need to return to the colony to unload.
When you release the bees at ‘A’ they fly at a particular bearing back to the colony. However, if you instead release them at ‘B’ they fly at a different compass bearing back to the colony.{{4}} .
How did the bees find the nectar-rich blue flowers in the first place?
Perhaps they observed another worker in the colony performing the waggle dance which informed them of the angle (from the sun) and distance to the blue flowers?
Alternatively, they might have just been searching around and chanced upon the blue flowers … they didn’t know they were there in the first place.
If they found the blue flowers by interpreting the waggle dance then you should be thinking how the waggle dancing bee found the blue flowers.
Alternatively, if they found the blue flowers by chance then you should be wondering how they will communicate their location to other foragers in the colony.
Transient nectar sources
Nectar sources are transient. They yield at particular times of the year … and of the day. The nectar may be dependent on recent rainfall or a variety of other environmental conditions.
All this means is that foragers may have to search widely to find a good source of nectar. If the source is really good – ample sugar-rich nectar and with lots of flowers producing it – then it’s important that the forager that found it tells her half-sisters how to also quickly find the same source.
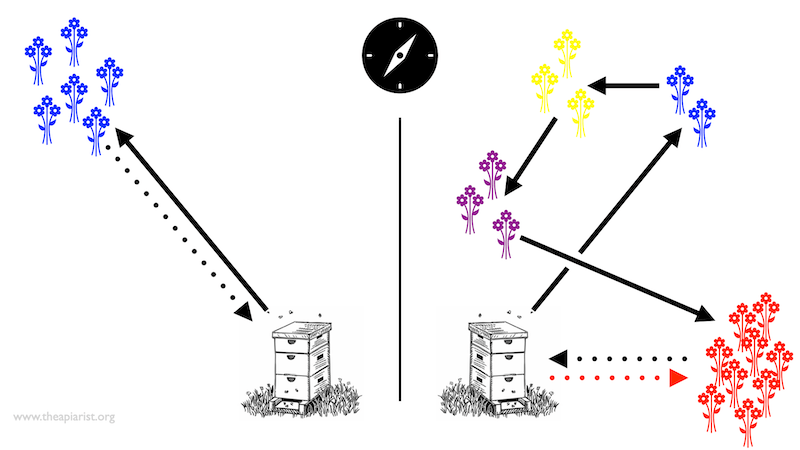
On the left the blue flowers have been yielding for days. The workers fly there in a straight line and return along the same path. Newly orientated workers observe the returning foragers waggle dancing and follow the same route to quickly and efficiently exploit the source.
But all good things come to an end …
On the right is what happens when blue flowers stop yielding. The foragers that arrive at the blue flowers find slim pickings and start casting about looking for a better source of nectar. They first find the marginally better yellow flowers, then the similar (but far from outstanding) purple flowers … so they keep looking.
And eventually, they find the red flowers. Lots of nectar and lots of flowers. They load up and return directly to the colony (black dotted line).
There are two striking things about this return flight. The first is that it does not follow (in reverse) the route by which they reached the red flowers. The second is that when these returning foragers perform the waggle dance they ‘instruct’ the observing bees to fly in the direction of the red dotted line … rather than to the blue, then yellow, then purple and then red flowers.
Path integration
The foragers who find the red flowers perform a process termed path integration to return:
Path integration is the process by which an animal, when moving away from a start point, often its nest, cumulatively sums its path, generating an internal vector that specifies the line from the animal’s current position back to the start point, however circuitous the outward trip (Collet, 2019).
This is a skill I singularly lack when trying to relocate my vehicle in the multi-storey car park.
Path integration is seen in other insects … Drosophila fruit flies can do it (over a range of centimetres), walking ants can do it over a range of hundreds of metres, and honey bees can do it over at least 5 kilometres (and probably more).
Path integration requires two pieces of information – the direction and the distance of travel.
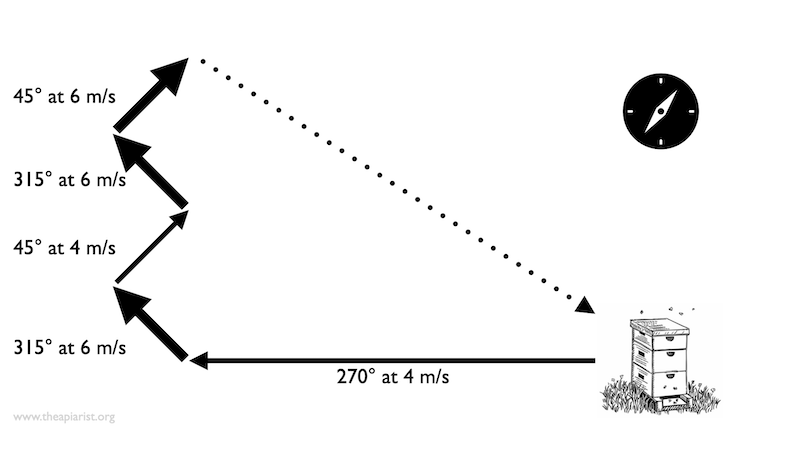
Path integration – individual parts of the flight are in different directions and of different lengths
Clearly, the very existence of the waggle dance provides compelling evidence that bees are aware of both. The dancing forager reports the angle (relative to the sun) of the nectar source and the distance at that angle that must be covered before the nectar source is located.
But for path integration, not only must the angle and distances be determined, they must also be cumulatively summed.
Neurophysiology and evolutionary conservation
Detailed neurophysiological experiments – recording the firing of individual neurones in the bee’s brain – have identified that these events occur in a region called the central complex (CX).
Two types of neurones are involved; the first is a set of polarised-light-based compass neurones and the second are optic-flow-based speed neurones. The former use celestial cues to create a visual compass. The latter provide a visual odometer (Stone et al., 2017).
Together – and there are additional integrator cells that link these functions – this relatively simple {{5}} neuronal circuitry allows path integration, enabling the bee to return ‘home’ directly after a convoluted outward flight.
Many of these studies were conducted on the nocturnal sweat bee Megalopta genalis. This forages at night when polarised skylight provides the directional cues in its rainforest habitat.
Importantly, similar neuronal organisation is found in the CX’s of locusts, some butterflies and dung beetles. The visual odometer neurones were analysed in Megalopta genalis, but are physically and likely functionally similar to structures found in Bombus terrestris (a bumble bee).
You may have noticed that none of these studies used our favourite, Apis mellifera, the honey bee.
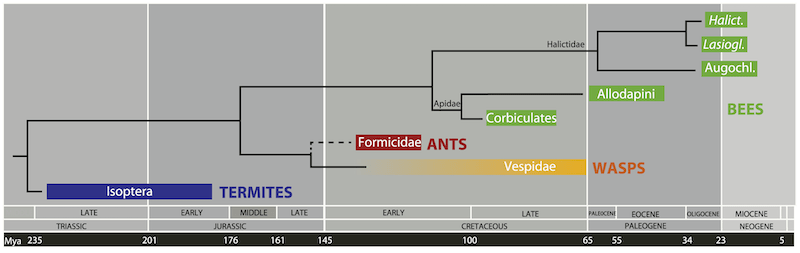
Nevertheless, there’s every reason to think that honey bee path integration involves very similar neuronal activity. Megalopta (belonging to the family Halictidae) and Bombus (a member of the Apidae family) are very distantly related and evolved from a common ancestor over 100 million years ago (Cardinal and Danforth, 2011). It’s therefore likely that all bees derived from this common ancestor – including honey bees – share similar neuronal activity underpinning their path integration ability.
Food vectors
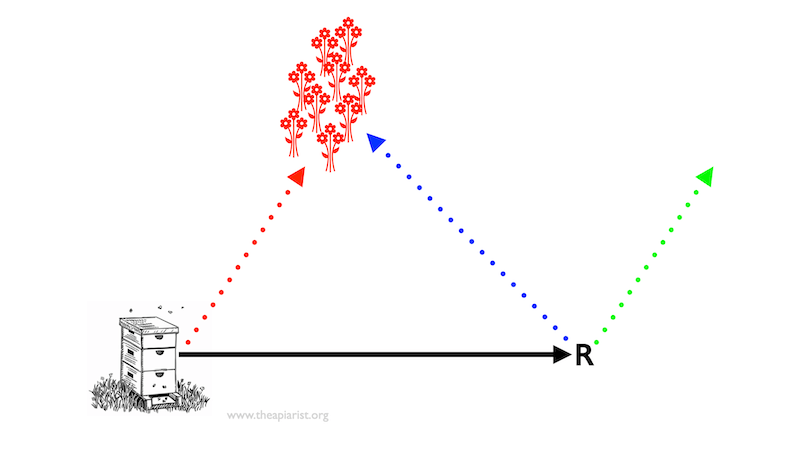
Before considering another point about honey bee flight I wanted to to briefly mention features of the outbound trip back to the high quality food source (the red dotted line in diagram above). This is termed the food vector and is essentially the reverse of the path integrated return flight back to the colony i.e. the same length, but pointing in the opposite direction.
The waggle dance communicates this food vector to nest mates of the successful returning forager.
But what happens if bees are displaced when starting, or while following this food vector?
For example, if a huge gust of wind blew them off course by tens or hundreds of metres, or an evil eager scientist captured them as they left the hive and transported them in a dark box across a couple of fields and then released them?
Do the bees fly a corrected route to the food source (the blue dotted arrow), or do they continue flying the same vector (angle and distance – the green dotted arrow) they would have done when they left the hive?
I’m not sure this exact experiment has been done with bees (but see below), but it has been done with ants (Cataglyphis fortis). In these studies the ’displaced’ ants did alter their direction of travel (Collett et al., 1999). The food vector is more than just an angle and distance, it also points to a position relative to the nest. The redirection exhibited by the ants was not perfect, but it clearly showed they were able to integrate the path to a location other than the nest after displacement.
Gusts of wind are not the same as eager scientists
However, back to the bees.
The gust of wind and eager scientist are not equivalent. Bees cope with gusts of wind every day. It always amazes me how well bees cope on windy days.
When blown off course they will get lots of visual cues – not least changes in optic flow and their angle to the sun – both of which should be readily corrected. If they didn’t then foragers would be lost in droves on windy days … or fail to find the food source.
In contrast, the eager scientist took care to place the bees in a darkened box, thereby immediately removing visual cues such as the angle of the sun and the optic flow.
In the studies conducted with the ants the scientists made sure the ants could see the sky but not the surrounding landscape (they trained them in open topped channels). This is because ants can also use landmarks in the surrounding landscape for orientation {{6}}.
And bees can do the same, which is the final sub-topic for this post on bee flight and orientation.
The map-like spatial memory of bees
Path integration is both useful and necessary. It means that foragers can return – fully laden – with minimum delay to the hive. They can therefore tell other foragers (via waggle dancing) promptly, and – in the case of elite foragers – they can set off again on another trip.
By reducing the distance flown – by integrating the path – they save not only time but ‘fuel’ as well i.e. path integration allows bees to maximise the nectar returned at the end of the foraging trip.
But, if all flights were a combination of random searches and path-integrated returns, why do bees go on orientation flights?
Orientation flights are short range (10’s to 100’s of metres) flights around the hive. These are taken by workers around 3 weeks after emergence as they transition form hive bees to foragers. They are also taken by older foragers if the hive is moved.
The very existence of orientation flights is compelling evidence that honey bees also use learned environmental landmarks for route finding, or at least for mapping the area around the hive to aid efficient return trips.
What evidence is there that these landmarks are used for this purpose?
Harmonic radar tracking of displaced foragers
I’ve previously discussed the use of short range harmonic radar to track bees ‘tagged’ with a small transponder. The key point is that it allows relatively accurate mapping of the entire flight of a bee up to 900 metres away. The resolution is, at best, about 3 metres.
Menzel and colleagues (Menzel et al., 2004) tracked the flights of three types of ‘displaced’ foragers:
- SF-bees trained to a stationary feeder a few hundred metres from the hive; these have ‘route memory’ and have traversed the route from the hive to the feeder multiple times
- VF-bees trained to a regularly moved feeder within 10 metres of the hive; these bees have no route memory
- R-bees which were recruited by a waggle dancing forager and have only secondhand route information of the position of the feeder i.e. they have never made the trip themselves
These are not trivial experiments. To ensure the environment was as uniform as possible they conducted the experiments in a large, flat mown field approximately 800 metres square. There was no forage within the field other than the experimental feeders. The field was surrounded on all sides by uniform coniferous woodland with insufficient variation in elevation (<1.5°) above the horizontal to provide any visual clues to the bees.
The field itself was not uniform. There were differences due to different mowing times and soil conditions. In addition, the scientists erected a number of radar-transparent coloured tents around the hive to provide additional landmarks.
Common features of flight paths determined by harmonic radar studies
Bees were allowed to orientate to the new hive position and then SF- and VF-bees were collected at a feeder and R-bees were captured as they left the observation hive (having ‘watched’ a waggle dance). The bees were fitted with a transponder, released some distance away from the feeder or the hive and then tracked by radar.
SF- and VF-bees were stuffed full of syrup and so – although they could fly for a long time – were motivated to return to the hive to unload their cargo. R-bees, whilst ‘primed’ to seek the feeder, had limited range and so would have to return to the hive to refuel.
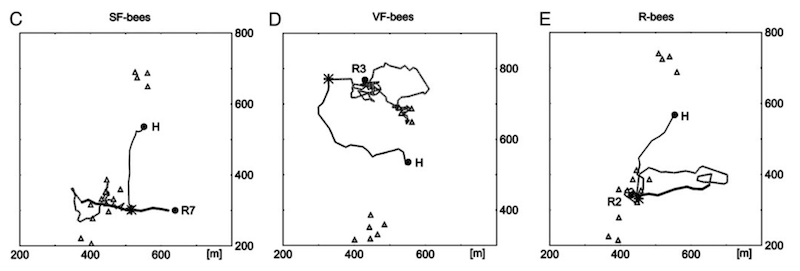
The SF- and R-bees exhibited three broadly conserved flight patterns during their return trip to the hive:
- A fast (20 m/s) straight line flight in the direction they would have taken back to the hive (for the SF-bees) or out to the feeder (for the R-bees). The length of this part of the flight was approximately the distance between the hive and the feeder.
- A slow (13 m/s) curved search flight.
- A fast homing flight back to the hive.
The VF-bees only exhibited the slow curved search flight and the final fast homing flight. This was unsurprising as they had never learned (or been told) to follow the route between the hive and distant feeder.
Food vectors and von Frisch
We therefore have the answer to the question I posed earlier (in the Food vector section above). A bee displaced when about to embark for the first time on a trip to a distant feeder – learnt from following a waggle dance – initially flies at the angle and to the approximate distance they would have taken from the hive (stage 1 of the flight).
Remember, unlike the ants, these foragers are ‘in the dark’ while being displaced, so have no visual clues about the displacement.
This is a really nice result and supports the contention made by von Frisch that the waggle dance communicates only distance and direction (relative to the sun) information, rather than positional information (von Frisch, 1967) {{7}}.
Homeward bound
After a period of slow curved flights the returning forager switches to a direct, fast homing flight. These started at positions – starred in the figure above – from which the bee could not see the hive (based upon distance and the known resolution of honey bee vision).
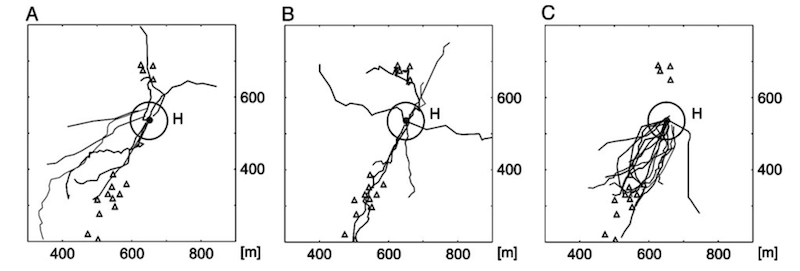
Homing flights of displaced SF-, VF- and R-bees (A, B, C respectively). H indicates the position of the hive.
Individual bees were randomly displaced around the study field. The homing flights were in a straight(ish) line and bees approached the hive from a range of different points of the compass. This argues strongly against the bees following a particular feature on the ground that led them back to the hive.
Instead, the authors argue that, since all the bees exhibit these direct homing flights, it must be based upon previous exploratory memory i.e. from orientation flights.
The tents were not critical landmarks. If they were moved some distance away the bees still returned using the same three flight phases (in the case of SF- and R-bees) and with similar navigational performance. Clearly there was sufficient information in the ground structure alone (mowing patterns, soil differences) acquired during the orientation flights.
In support of this, some of the harmonic radar data showed bees flying along boundaries between mown areas (in a similar way to homing pigeons follow rivers or motorways; Guilford and Biro, 2014.).
These experiments indicate that during orientation flights the bee develops a local spatial memory of landmarks that provide a ‘memory map’. This enables the bee to return to the nest once it recognises some of these familiar landmarks.
Repeated displacement flights of the same bee further indicated that the landmarks recognised (whatever they were) could be approached from different angles.
Final inspections
My bees are still out foraging despite the large blocks of fondant most hives are now topped with. I’m not sure what they’re collecting but it’s clearly worth the trip … and going to the initial trouble of finding it and telling other foragers about it.
We usually take the amazing navigational abilities of our bees for granted. Those returning foragers are using navigational skills that evolved at least 100 million years ago while dinosaurs roamed the earth.
100 million years is a long time to develop a range of skills and subtleties; it’s no wonder we still only partially understand honey bee navigation. Of course, we don’t have to understand it to still marvel at their ability to find the way back.
And it’s worth also remembering that these navigation skills – many of which are based upon the angle of travel relative to the direction of the sun – also operate on dull, overcast days. But that’s a topic for another post …
References
- Cardinal, S. and Danforth, B.N. (2011) ‘The Antiquity and Evolutionary History of Social Behavior in Bees’, PLOS ONE, 6(6), p. e21086. Available at: https://doi.org/10.1371/journal.pone.0021086.
- Collett, M., Collett, T.S. and Wehner, R. (1999) ‘Calibration of vector navigation in desert ants’, Current Biology, 9(18), pp. 1031–1034. Available at: https://doi.org/10.1016/S0960-9822(99)80451-5.
- Guilford, T. and Biro, D. (2014) ‘Route following and the pigeon’s familiar area map’, Journal of Experimental Biology, 217(2), pp. 169–179. Available at: https://doi.org/10.1242/jeb.092908.
- Menzel, R. et al. (2005) ‘Honey bees navigate according to a map-like spatial memory’, Proceedings of the National Academy of Sciences, 102(8), pp. 3040–3045. Available at: https://doi.org/10.1073/pnas.0408550102.
- Stone, T. et al. (2017) ‘An Anatomically Constrained Model for Path Integration in the Bee Brain’, Current Biology, 27(20), pp. 3069-3085.e11. Available at: https://doi.org/10.1016/j.cub.2017.08.052.
{{1}}: Truth be told there are some technical issues with this … some days it never arrives at all, then two arrive the following day. A few months ago it went offline for 2-3 weeks and then flooded my inbox when service was restored.
{{2}}: A little over 1600 years by my calculations, ignoring the fact that the dictionary is growing by about 3000 new words every decade or so.
{{3}}: I can’t use ‘hive’ here as it’s really a way of finding feral bee nests.
{{4}}: Although this is how beelining is described in some books there are issues with this particular approach that will become more obvious if you read on.
{{5}}: I’m using ‘relatively’ in a very general sense here.
{{6}}: Of course, this also meant that displacing the channel still allowed the bees to determine the displacement while traversing it.
{{7}}: We also now know that it also includes information on the quality of the nectar or pollen source.

Join the discussion ...