Idle thoughts and busy foragers

It's been a busy week.
Somehow or other I misinterpreted the instruction “put your feet up, it's the end of the beekeeping season”, with “ask the shops whether they need more honey before Christmas”, and was swamped with orders.
Consequently, I've spent the week preparing, jarring and labelling honey.
It's always nice to sell some honey. Even ignoring the percentage (no doubt shortly to increase 😞) that the Chancellor of the Exchequer takes, the income more than helps to offset the costs of my hobby beekeeping.
But, because it's a hobby, it is by definition relatively small scale.
I mentioned last week that there's no Swienty extraction line in my honey processing room. Similarly, I don't have a DANA api MATIC filling station and turntable {{1}} or an automated labelling machine.
It's all done manually, with a bit of help from my honey bucket tipper and a 3D printed labelling aid.
The first of these makes jarring easier (which is not exactly the same as easy). Commercial versions have become available since that post first appeared 11 years ago. The labelling aid 'simply' ensures that every label is horizontally and laterally aligned {{2}}, meaning the jars look good on the shelf.
Does it help sales? Who knows? Presumably, because there are lots of repeat orders.
Anyway, with those simple aids, a couple of dozen jars are a breeze, and things only start to get tedious after about a gross.
Much more than that and my mind starts to wander.
Sponsors get more … posts, news, and information on the science, art, and practice of sustainable beekeeping. They also have access to over a decade of legacy posts, and ensure The Apiarist continues to appear every week.
Idle thoughts
In fact, my mind has probably been wandering well before I've passed the gross mark.
I'm easily distracted.
One perennial problem I think about is what to deliver the jars in to the shop.
Jarred honey is heavy. Filled 227g and 340 g jars weigh ~410 g and ~575 g respectively. An order for 60 of each therefore weighs about 60 kg (i.e. the weight of a smallish beekeeper). You need stout cardboard boxes to carry whatever proportion of that weight I can manage to get from the boot of the car to the shop counter.
Most cardboard boxes are not strong enough, and the last thing I want is to make a disastrous 'curbside delivery', leaving 50 smashed jars on the pavement {{3}}.
The obvious thing to use are strong, stackable, plastic storage crates. Unfortunately, my experience is that — however clearly labelled — any left with the shop to be “collected when I next visit” are never there when I return 😞.

I have recently discovered that plastic fish crates (as used on trawlers, or salmon farms) are the perfect size to take 40 × 340 g jars {{4}}. That's about the maximum I can uncomfortably carry, so at least it reduces the trips from the car to the shop. I used to find these crates when beachcombing on the West Coast, and they've languished behind the shed for the last couple of years.

Of course, having now realised how useful they are, I live miles from the sea 😢.
I currently use a variety of plastic boxes for deliveries, and transfer them to empty cardboard boxes in the shop (small enough to be safely lifted by the staff) while chatting about how business is. Perhaps surprisingly, considering the state of the economy and the number of vacant premises on the high street, the farm stores I supply seem very buoyant about customer numbers and sales.

Labelling thoughts
I print my own labels using black on white thermal paper and a Brother QL-800 printer. I've mothballed my original Dymo LabelWriter as every new version of the software was incrementally (and often much) worse than the last, and the most recent variants of the printer only work with Dymo-brand paper.
Brother have no such restrictions, though I always use Brother-brand paper because it's appreciably better quality than any of the copy-cat brands I've tested. I'd prefer to have the choice, rather than be forced to buy a particular brand of labels. If another supplier starts producing better paper, or different sized/shaped labels, I'd like to be 'allowed' to use them.

I use Brother's DK-11201 address labels (29 × 90 mm) for the front of the jar, with a 23 mm square label on the reverse carrying the batch number, best before date, and a QR code linked to information on the provenance of the honey. Some jars also get a 'Local honey' roundel label on the lid.
I recently prepared and labelled some honey wedding favours in very small hexagonal jars. All the pre-cut roll labels were too long, wrapping almost completely around the jars. However, Brother also sells continuous labels (in a variety of widths × 30 metres), which allowed me to print a narrower label, cut to length using the printer's internal guillotine.
With new honey to label from the Scottish Borders my wandering mind is also wondering whether a single thistle (indicating 'Produce of Scotland') could be misconstrued by Trading Standards as an indication of the monofloral source of the nectar. I've seen some egregious breaches of the honey labelling regulations, and barely ever heard of anyone falling foul of them … but, knowing my luck 😞.
More on provenance
Local honey sells well. When I used to sell from the door, there would always be questions about where the bees were, what were they foraging on, or how far they forage?
Selling through a third party (a shop) means that personal contact, and the information, is missing.
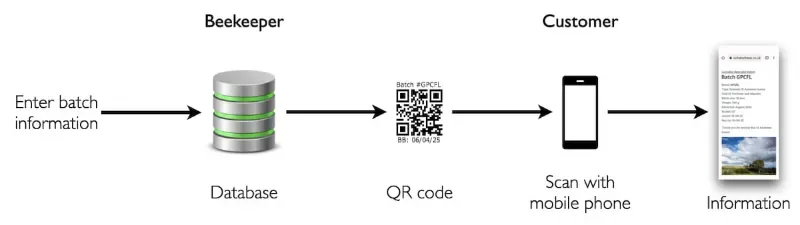
The QR codes I add to each jar go some way to provide this missing information. Each links to a webpage that has details of the honey, the area the bees are in, the likely forage types at different times of the year etc. It works reasonably well, and a proportion — relatively small, but worthwhile as far as I'm concerned — of customers read up about the honey they have just purchased (or that they're considering buying … they just need to scan/photo the QR code in the shop).
However, the generation of the QR codes, and what a programmer would call the 'back end' i.e. everything that the customer does not see, is a bit kludgy (or, what a programmer would call, 'complete rubbish').
So, while going through the tortuous repetition of front label, turn the jar, anti-tamper label, QR/batch label, local honey label … my wandering mind is thinking up ways to streamline things, and to make the system a bit more usable.
I've got a cunning plan. Whether I'll have a chance to implement it rather depends upon how many honey orders I get before next season starts.
Sponsors ensure the continued existence of the site, and receive regular sponsor-only content. This is a ChatGPT-free zone. ChatGPT has never opened a hive or labelled a jar of honey, so why trust its judgement when seeking information or entertainment on the science, art, and practice of sustainable beekeeping?
Forage, foragers and foraging distances
But, while we're on the subject (and I sort of was), how can you determine what the bees are foraging on?
If it's mid-May, and the hives are sitting in a field margin with acres of dazzling yellow oil seed rape blooms as far as the eye can see, then it's self-evident.

The resulting honey will be high in glucose, and will readily crystallise. If you leave the supers on too long it will set rock hard in the frames, creating a wobbling-wonderland if you try to extract it conventionally {{5}}. The pollen baskets on returning foragers will, unsurprisingly, be bright yellow.
Later in the year, if the lime is flowering, and you can hear the bees working the trees from 100 metres away, then it's again a 'no-brainer' (but make sure it's not just bumblebees and wasps).
Similarly, hives on the moor in late August should be concentrating on the abundant purple heather, not the dribs and drabs of other things they might find.
But what about other times of the season?
For example, what about late summer, after the main flow is over? That all-important time of the year when the bees are starting to make their preparations for winter.
As nectar and pollen availability starts to wane, what can the bees find, and where do they find it?
In the discussion that follows its worth remembering that nectar is likely to contain pollen from the plant it was collected from. If we want to know what nectars they are collecting we need to analyse the honey stores, but if we want to more generally determine where the bees are foraging for protein (pollen) we can directly analyse the pollen collected.
Barcoding
If I wanted to know what the bees were collecting I could watch bees arriving at the hive entrance, do some pollen analysis by microscopy (from honey), or I could spend a chunk of cash from my honey sales and use DNA metabarcoding (on the pollen in honey, or pollen directly).
The beauty of the latter approach is that it looks at the entire spectrum of pollen collected. If you've sent samples off to the UK's National Honey Monitoring Scheme (NHMS) you might be familiar with this sort of analysis.
The DNA is extracted from the sample submitted, amplified to produce a machine-readable signal which is then identified by sequence analysis. The method offers three very significant advantages:
- Results are qualitative and quantitative. You can determine what the bees have been foraging on, and the relative proportion of pollen collected from each species.
- The method is sensitive. If a sample contains 5% dandelion pollen and 95% oil seed rape {{6}}, you'll still be able to detect the former. Using microscopy you might not even notice the dandelion pollen.
- It's a high-throughput method, which means that dozens or hundreds of samples can be analysed simultaneously, thereby both saving time and avoiding the need to repeat internal controls for standardisation and comparisons.
What's not to like? {{7}}
The NHMS programme involves annual (or perhaps biannual) sampling and analysis from ~1000 apiaries.
The same approach, over a shorter timescale (weeks rather than years) and a smaller geographic area, has recently been used to provide insights into what the bees are foraging on as summer segues into autumn.
Pollen diversity and pesticide exposure
An interesting study was published last month (Manzer et al., 2026; it's online well before it appears in the printed journal) showing the sorts of results that this type of metabarcoding analysis can produce.
The authors analysed pollen (from pollen traps, not honey) from 36 hives in 9 locations near Würzburg in Germany, selected on the basis of the amount of arable cropland — ranging from 43% to 97% in land coverage — within 2 km from the apiary.
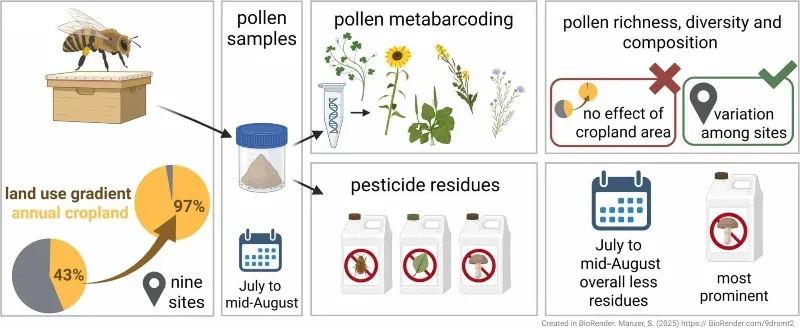
In each case, pollen was collected between July and mid-August, sampling every third day. The pooled pollen sample was subjected to metabarcoding to identify the relative amounts of each type of pollen present. In addition, the authors also analysed pesticides in the pollen.
The expectation was that intensively farmed areas would show a reduced overall diversity of pollen, and reduced pollen species turnover (i.e. less change from week to week), coupled with higher pesticide residues than areas with less arable cropland (from Manzer et al., 2025).
Since pollen diversity is a 'good thing' (providing a rich and varied diet for the bees, including a full spectrum of the necessary amino acids and sterols {{8}}), and pesticides are the exact opposite, if these expectations were met it would suggest that intensively farmed arable regions were measurably worse for honey bees.
Yes, you might get 50 kg of honey per hive when the OSR was flowering, but the bees might subsequently go into winter poorly nourished and with a range of physiological issues associated with elevated pesticide levels.
This is just the sort of thing that could contribute to high winter losses.
So, were these expectations met?
In a word, no.
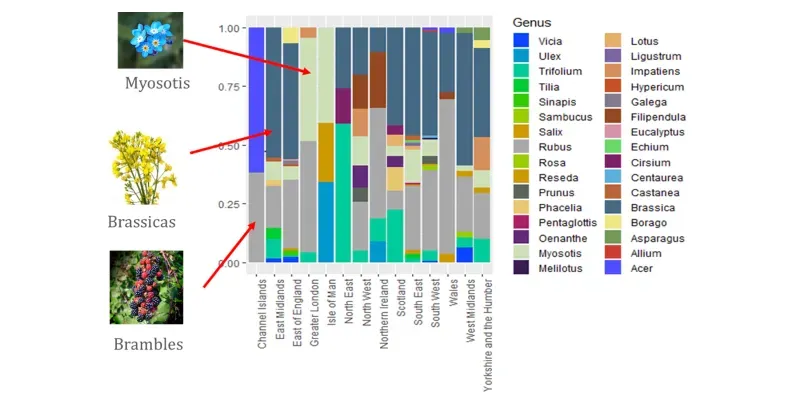
Pollen diversity — so-called species richness — did not correlate with the area of arable cropland within 2 km of the study apiaries. In fact, irrespective of the percentage of arable cropland within 2 km, the diversity was remarkably similar across all the survey sites. In addition, the rate of pollen turnover, was also unrelated to the amount of arable cropland.
That's not to say that there weren't differences between sites.
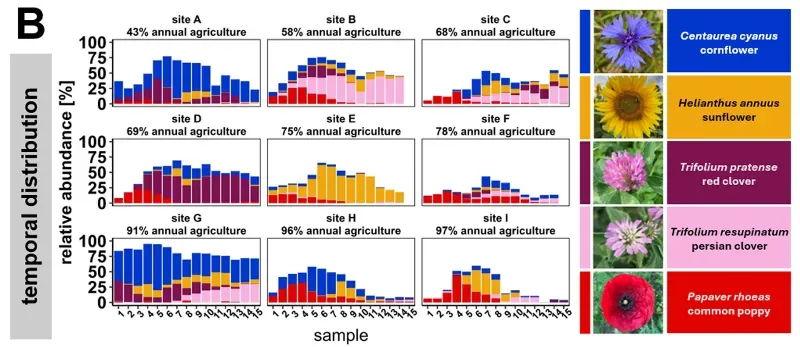
There were, and these differences were often quite large. A total of 140 pollen species {{9}} were identified, of which 93 comprised more than 1% of the total in the analysis (remember, I said earlier that the method is quantitative and qualitative, and sensitive). Of these 93 species, the three most abundant collectively represented over ~40% of samples identified. However, these varied significantly from apiary to apiary:
- Red clover (Trifolium): ~17% of total (range 3–35% per site)
- Cornflower (Centaurea): ~14% (range 3–45%)
- Sunflower (Helianthus): ~8% (range 1–30%)
On average, each 3 day sample contained ~10 different pollen species, though these might differ from the species found in the following or preceding 3 day sample from the same location.
Clearly, the bees thoroughly work the environment for suitable pollen sources. The environment is dynamic, and the bees are capable of exploiting the rapid changes that occur in pollen availability.
Pesticides
The other expectation not met was the pesticide levels within the pollen. Only 39% of samples contained detectable pesticides {{10}}, with fungicides being the most commonly detected. Pesticide levels rapidly dropped after the end of July, and appear to correlate with sunflower pollen abundance in the samples. Pesticide levels broadly correlated with the area of cropland, with more detected in the more intensively farmed areas.
Is this good or bad?
As I now live in a rich agricultural area, I found these results reasonably encouraging. It appears as though the bees — at least in that area of Lower Franconia, Germany — are good at seeking out and collecting a wide range of pollen species.
Although the Scottish Borders (or wherever you live) undoubtedly differs from Southern Germany, the bees are essentially the same.
Pollen varies in its protein content, and the bees have evolved to collect what they need. They are very good at both exploiting abundant pollen sources, and finding the additional pollen types needed to compensate for the less good pollens in the environment.
Extrapolating meaningful conclusions from the pesticide detection, relevant to my bees and area, is more difficult. What's approved and where it's approved varies, and the crops that the pesticide is approved for (in Germany) may well not be grown near me. Or you.
Additional considerations
Let's deal with the fungicides first, as I suspect these are a 'local' issue, linked to both location and year. The field work was conducted in 2021, with 23 days of rain in July and August. Fungicides are often used protectively in warm and humid conditions, so it is possible that fungicide levels would be lower in a drier year (or region).

Secondly, there was a correlation between the two fungicide detected (fludioxonil and azoxystrobin) levels and sunflower pollen. Of these, one is only approved as a seed treatment for sunflowers, and the other — although, by definition, detected when sunflowers are flowering — is not approved for use on sunflowers.
From the data collected, it's not possible to determine whether the presence of these fungicides was due to bees foraging on sunflower-adjacent crops (or wild flowers in field margins) where use was approved, or off-label usage.
Finally, on this point, there's relatively little acreage of sunflowers grown in the UK, as discussed in an earlier post about low Varroa levels correlating with sunflowers.
Foraging distances and areas
The second point is probably more significant, and relevant wherever your bees are. This is the choice of a radius of only 2 km from the hive when looking at land usage.
Surely bees forage further than that?
They do, but they probably don't unless they have to.
There's an energetic cost to foraging that must be justified in terms of what's being collected. There's little point in flying 5 km for nectar with a very low sugar content, or a similar distance for a pollen that's available in limitless amounts over the fence.
However, during a dearth — of pollen or nectar — or in impoverished landscapes, bees will forage further than 2 km. Previous studies, including from these authors, have shown that foraging distances increases when resources are scarce (e.g. Steffen-Dewenter & Kuhn, 2003, Beekman & Ratnieks, 2000). It's therefore possible that the relatively high diversity of pollen collected in the Manzer et al., (2026) study may mean that the bees were travelling outwith the 2 km circle from the hive, potentially into much richer floral environments.
I will revisit the Steffen-Dewenter & Kuhn (2003) study again in a future post.
And finally
Of course, when I'm asked by a customer “How far do the bees fly to collect nectar?” I'll make sure the answer is a lot easier to understand than it appears from the scientific studies, where energetics, waggle dance interpretation, rare sterols in specific pollens, and landscape complexity are a consideration.
I usually say “2–3 km”.
And, my fancy little QR code — on the back of all those dozens and dozens of jars packed up ready for delivery in fish crates — links to a web page with a simple circle drawn on a map.
Of about 2.5 km 😄.
The KISS principle
More like this?
That's over 3,000 words you've just read on bees and beekeeping. If you want more of this every week, then why not sign up as a sponsor of The Apiarist? At least 50% of posts are now for sponsors only, and sponsors ensure that there will be comprehensive, well researched and entertaining posts on sustainable beekeeping appearing every week.
Notes
I'm all talked out. I'm reducing the number of talks I give next winter, to allow me some time to label yet more jars prepare some new talks on a range of beekeeping topics. If your association wants to book me for a talk in 26/27 then please note the limited dates available on my 'talks' website. Normal service will be resumed, but not until the 27/28 winter season. I am already fully booked for this winter.
References
Beekman, M., and Ratnieks, F.L.W. (2000) Long-range foraging by the honey-bee, Apis mellifera L. Functional Ecology 14: 490–496.
Manzer, S., Sponsler, D., Keller, A., Honert, C., Brühl, C.A., Mainardi, G., et al. (2026) Effects of annual cropland and season on pollen diversity and pesticide exposure in honey bee colonies. Agriculture, Ecosystems & Environment 396: 109987 https://www.sciencedirect.com/science/article/pii/S0167880925005195.
Steffan-Dewenter, I., and Kuhn, A. (2003) Honeybee foraging in differentially structured landscapes. Proceedings of the Royal Society of London Series B: Biological Sciences https://royalsocietypublishing.org/doi/10.1098/rspb.2002.2292.
{{1}}: If you've got a lot of honey to jar, don't watch the promotional video as it'll break your heart. And, if you buy one, it'll break your bank 😉.
{{2}}: My jars are square, and the label wraps around the sides a bit, hiding the boring stuff like addresses, so emphasising the things I want to, and keeping the focus on the honey. However, I've built similar labelling-levellers for round and hex jars.
{{3}}: Speaking of curbside deliveries … a big shout-out (of the “WTF were you thinking?” type) to the driver who asked this week for help lifting a 200 kg pallet of jars off the back of his truck, having chosen to deliver them in a van without a tail lift or a pallet truck. Muppet.
{{4}}: At least of the design I use … YMMV as they say.
{{5}}: Time to buy another expensive piece of kit … a DANI api MELTER.
{{6}}: Which is usually reported as Brassica, as the sequence of turnip and OSR are near-identical in this type of analysis.
{{7}}: Well, the £45,000 for the DNA sequencer is one thing, and the high cost of reagents to do the analysis is another 😉.
{{8}}: Monofloral nectar and pollen sources are known to be detrimental to colony health as they don't necessarily contain all the components — micronutrients, rare sterols, particular amino acids — needed by the bees.
{{9}}: Actually genera, as it's sometimes not possible to discriminate between individual species using DNA metabarcoding. In a previous footnote I've mentioned Brassica rapa (turnip) and B. napus (OSR) as being indistinguishable. Likewise, bramble — which evolves very rapidly — just appears as Rubus.
{{10}}: The authors designate these 'current use pesticides', which I take to mean that others might be detectable, but are not currently in use, although they still contaminate the environment.
Join the discussion ...