Hype or hope?
Synopsis : How promising is the “world’s first vaccine for honey bees”? Separating hype from hope, good news from bad news, and with a bonus discussion of trans-generational immune priming and beemageddon.
Introduction
Bad news sells newspapers. A survey of two decades of news preferences by the Pew Research Centre showed that the most-read stories were those that could be classified as either war, weather, disaster, money or crime.
’If it bleeds, it leads’ as they say.
And combinations – like crooks making huge profits from pandemics – ensure top billing.
Enough! This isn’t a politics blog … let’s move on.
That survey was ~15 years ago, but it’s equally true today. However, these days additional topics sometimes force their way into the buffet of gloom that make up the headlines. Royalty is one, particularly with the tabloids, though sometimes these could equally well be classified under ‘war, weather, disaster, money or crime’ {{1}}.
More recently, climate change and environmental apocalypse have started headlining. Unfortunately for journalists, the measurables are often rather esoteric. Kilotons of greenhouse gases or atmospheric CO2 levels of over 400 ppm mean little to the layman, or most journalists.
But cute, furry, hard working bees do … particularly if there’s no mention of stings 😉 .
So, the impact of climate change or the environmental apocalypse on bees often gets top billing … and not just any bees, honey bees.
Everyone knows what a honey bee is, and almost everyone loves honey.
Beemageddon
All of which means that some of these stories are couched in terms of the potential for beemageddon. And because honey bees are ‘threatened’ {{2}} and familiar, related stories about honeybees also tend to get wide coverage.
Just recently we’ve had ancient bees in Oxfordshire woodland {{3}}, the danger of almond milk {{4}} and the world’s first vaccine for honey bees. These are all from The Guardian … I searched the Daily Mail as well {{5}} and they covered the reduced lifespan of bees which I might discuss in a future post {{6}}, some outstanding work on CBPV 😉 and the Royal beekeeper informing the bees of the death of Her Majesty.
Royalty, death and bees? BINGO … we have a winner!
One of these – the bee vaccine – looks like it could be good news.
Of course, the need for the vaccine means there’s some bad news to fully justify the story (don’t forget that impending beemageddon).
The world’s first bee vaccine sounds good doesn’t it?
Let’s have a look at some of the claims and the technology behind it.
Vaccines
What is a vaccine?
In the dim and distant past the word vaccine originated from the Latin for ‘cow’ (vacca). The link to vaccines is of course Edward Jenner who made a smallpox vaccine derived from the related cowpox virus.
In 1798, in an experiment that would shred all current ethical regulations, Jenner inoculated James Phipps with cowpox and, six weeks later, challenged him with pustular material from a smallpox case. Not only did Phipps survive, but he developed no smallpox symptoms either.
A vaccine is therefore something derived from an infectious agent that, when administered (inoculated), provides protection (immunity) from subsequent infection (challenge) by the same or a closely related infectious agent.
As an aside, Jenner could perhaps claim to have made the world’s first vaccine … despite the fact that a Dorset farmer, Benjamin Jesty, had done the same thing two decades earlier. In addition, variolation, usually involving administering dried smallpox material from a mild case, had been used prophylactically to prevent smallpox for hundreds of years.
Claiming originality is a tricky business … and something I’ll return to.
In mammals (like James Phipps) vaccines work by primarily stimulating a cell- and protein-based immune response. Cells are ‘primed’ to recognise a pathogen. Should subsequent infection occur, the cells and proteins, which have a molecular memory, are reactivated, amplified and subsequently destroy the invading pathogen.
Similar immune systems have proven so effective that evolution has created lots of them … many of which bear little immediate similarity to the one mammals (and most vertebrates) have. But there are similarities; many also have a ‘memory’ that can be reactivated should subsequent exposure occur.
And, scientists are starting to understand immunity in honey bees, and how to exploit it.
Bees, flies, RNA and, er, dunno
The most detailed studies of insect immunity have been done with fruit flies (Drosophila) as they are, by some distance, the best understood insects. The primary immune system in flies is not protein based, but instead uses nucleic acids. Immunity involves things that are called interfering ribonucleic acids which, understandably, are conveniently abbreviated to RNAi.
And honey bees also have and RNAi-based immune system, and it is increasingly well understood. Actually, it’s so well understood that we can stimulate it with experimental treatments against Israeli Acute Paralysis Virus and Deformed wing virus or even Varroa.
‘Experimental’ in this case means ”works, sort of, but not as well as we want … or need”.
But the world’s first bee vaccine that I’m going to discuss this week is not RNAi-based. It uses a completely different immunity system termed trans-generational immune priming, which is also conveniently abbreviated to TgIP (or TGIP in some studies {{7}} ).
And the thing about TgIP is that we have almost no idea as to how it works.
Just like the press article (“New vaccine to prevent beemageddon”) there’s both good news and bad news resulting from our cluelessness about TgIP.
The good news is that I can focus on the results rather than the mechanism, so making this post precisely 6430 words shorter than it would otherwise be.
The bad news is that by focusing on the results and some of the subsequent claims, the balance between the hope and hype in the title of this post gets shifted a bit 🙁 .
Sorry.
The discovery of TgIP in honey bees
The Tg (trans-generational) in the TgIP reflects the fact that:
‘ … maternal immune experience has been demonstrated to be transmitted to progeny and may therefore have a positive impact on offspring resistance and survival’ (Hernandez Lopez et al., 2014).
Essentially that means that if the mother is inoculated, the ‘children’ inherit some of the immunity.
Although the mechanisms are usually poorly understood – and may well not be the same in all species – TgIP has been demonstrated in vertebrates and invertebrates, including amongst the latter, bumble bees (Sadd et al., 2005), beetles and butterflies (Tidbury et al., 2010; Freitak et al., 2014).
The first study I’m aware of on honey bees was conducted in the University of Graz, Austria (Hernandez Lopez et al., 2014). In this study they demonstrated that queens immunised with the causative agent of American Foulbrood (AFB) subsequently produced larvae that were at least partially resistant to subsequent AFB disease.
American foulbrood
American foulbrood is a bacterial disease of honey bees caused by Paenibacillus larvae. The name, foulbrood, reflects the smell of diseased larvae in the hive. It is a brood disease, caused by the ingestion of bacterial spores by very young larvae. The spores are effectively metabolically inert, heat resistant forms of the bacteria, that can survive for decades and germinate when ingested by a larva.
Adult bees are resistant to infection, but are the vectors that transmit the spores between larvae in the hive, and between hives while drifting and robbing.
AFB outbreaks are dealt with by destruction of the colony and in many countries it is a notifiable disease.
Inoculating queens by injection
Hernandez Lopez and colleagues heat-killed (90°C for 10 minutes) a vegetative {{8}} culture of Paenibacillus larvae and injected mated queens with the resulting ‘soup’ of proteins. They subsequently returned the queens to their nucleus colonies and, at an unspecified time later, removed day old larvae to plastic dishes in an incubator and fed them an artificial diet containing infectious P. larvae spores.
Then it was simply a case of counting the corpses …
Like all proper experiments this one was controlled in a variety of ways. To determine whether any benefit observed was due to inoculation of the queen with the heat-killed bacterial soup they injected a similar number of queens with an inert buffer solution. They repeated the study a year later and used two different strains of P. larvae spores for the challenge.
Finally, they tested the colonies both before and after inoculating the queens. This was an important control. Queens were unrelated and open mated and this control was needed to demonstrate whether there was any inherent differences in susceptibility to AFB, for example reflecting genetic differences between larvae.
For brevity, I’ll only show one set of the results.
Counting the corpses
Results from these type of studies can be presented as dull-as-dishwater numerical tables, or visually-rewarding ‘kill curves’. More properly these are called Kaplan-Meier curves. These plot time (horizontal) against the proportion of test subjects that exhibit – typically – disease or survival (vertical).
The resulting stepped curves therefore start at 100% (sometimes – as here – represented as 1) and drop over time as the corpses pile up. The stepped curves plot the cumulative body count.
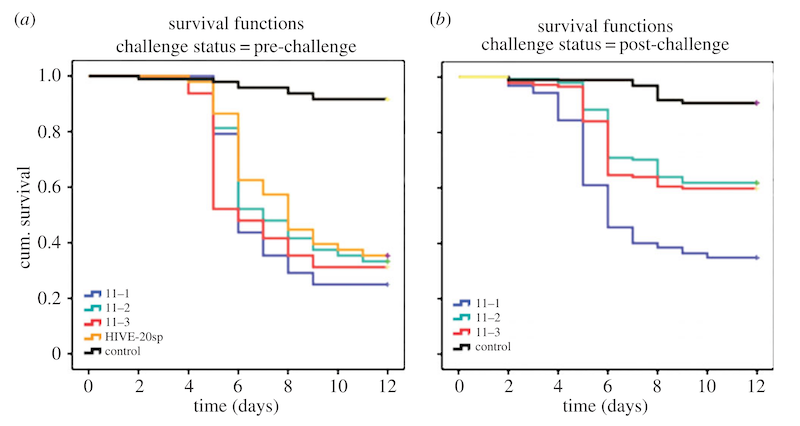
Kaplan-Meier plots of larval survival a) before queen vaccination, b) after queen vaccination (11-2 & 11-3)
The black line indicates larval losses due to experimental handling. These control larvae were not challenged with AFB. As you can see, about 5-10% of larvae die off during the experiment and it’s nothing to do with AFB.
The graph on the left (a) shows larval survival after AFB challenge before the queens were inoculated. There’s no statistical difference between the coloured lines indicating that all are equally susceptible to AFB infection.
On the right are the kill curves Kaplan-Meier curves after inoculation of queens with the control buffer (line 11-1) or with the heat-killed P. larvae bacterial soup (lines 11-2 and 11-3). Clearly fewer larvae die over the time course of the experiment from nucs headed by inoculated queens.
Remember, these are cumulative survival curves – each line is derived from 96 to 192 individual challenged larvae.
Overall 65% of challenged larvae from buffer-inoculated (11-1) queens succumbed, whereas only 39% died after challenge if the queen had received the P. larvae ’soup’ inoculation.
Therefore this demonstration of TgIP in honey bees accounts for a reduction in larval mortality of ~26%.
You won’t feel a thing
Whilst broadly encouraging, there are a couple of issues with the Hernandez Lopez study that limit its application or potential usefulness.
The first is the level of protection seen. The dose of spores administered to the larvae was small, and potentially much smaller than one naturally experienced by larvae doing in-hive infection.
How good would the protection be with a dose 10, 100 or 1000 times as large? This can be tested, and presumably white-coated boffins with ample foreheads are busy doing this as I write.
The second is that the queens were inoculated with the P. larvae ‘soup’ by direct injection. This involves chilling the queen on ice for several minutes, injecting her using a sterile syringe, warming her up and then returning her to the colony.
Inevitably a few queens are lost during this process and – at least with workers – there are some issues with longevity for bees that have been cold-anaesthetised (though I can’t remember seeing any data on queen longevity following this procedure).
Injecting queens is practical if you want a few dozen, but it’s a non-starter if you’re producing thousands or need tens of thousands.
For those sorts of numbers you need to feed the queen with the vaccine … which, since mated queens don’t feed themselves, means caging the queen with a few workers and providing a food source laced with the vaccine.
A recent attempt to induce TgIP against European foulbrood (EFB) by feeding the queen with inactivated Melissococcus plutonius (the bacteria that causes EFB) was unsuccessful (Ory et al., 2022).
Perhaps oral induction of TgIP is a non-starter?
A glimmer of hope
However, towards the end of 2022, the University of Graz group published a study demonstrating promising evidence for TgIP using orally administered Paenibacillus larvae (Dickel et al., 2022) and this is the basis for the world’s first bee vaccine.
The paper was published in Frontiers in Veterinary Science and is relatively light on data.
This isn’t unusual for a scientific publication supporting a commercial – or planned commercial – product. The focus is on safety and demonstrating some level of efficacy, rather than understanding the underlying mechanism.
Or for that matter providing too many details that could undermine its potential commercial success, or the attractiveness of the company to future investment.
Bacterin = ’soup’
The vaccine preparation was commercially prepared by the company involved in the study (Dalan Animal Health) and there are no details of how it was prepared or what it contains. It could just be a heat-inactivated bacterial soup, or it might be supplemented with all sorts of weird and wonderful ingredients like unobtanium or virgin unicorn tears.
If you ask them it would probably be a case of ”I could tell you, but then I’d have to kill you” {{9}}.
The authors use the term bacterin (you won’t find this word defined in the OED … though unobtainium is in there). If you’re reading the Dickel paper just remember to translate bacterin to soup.
Let them eat cake bacterin
Caged queens, with attendant workers, were fed soup bacterin added to a corn syrup/sugar mix for 8 days and then used to requeen 5 frame nucs {{10}}.
After at least 18 days, day-old larvae were harvested from the nucs, transferred to the incubator and challenged with a stock preparation of AFB spores in larval food. Larvae were subsequently fed AFB-free larval food and monitored for a further 8 days of development.
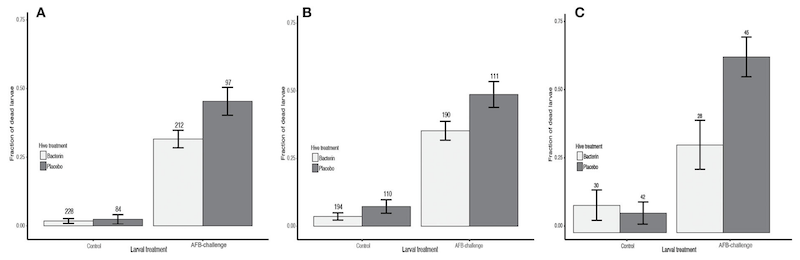
The experiment was repeated 3 times in two different study sites. In the interests of providing the bare minimum number of results (!) the authors simply plot the proportion of larvae derived from placebo- or bacterin-fed queens that died over the 8 day period.
Unfortunately, the axis labelling on the graph is almost too small to read in the original let alone the image above.
This figure shows three repeats. Larvae from bacterin- and placebo-fed queens are in light or dark shading respectively. For each repeat, paired bars on the right are the AFB-challenged larvae.
Note that the bacterin-fed larvae are shorter bars i.e. fewer died.
Overall, ~50-60% of larvae from placebo-fed queens succumbed to AFB, a figure that was reduced by 28-30% following prior vaccination of the queens with the bacterin.
‘By’ not ‘to’ 28-30%.
The results were statistically significant but perhaps less good than you’d hope for.
An approved vaccine for AFB …
Well, to be pedantic, the approval that has been issued is a conditional licence by the USDA Animal and Plant Health Inspection Service. There’s a PDF with very few details on their woefully slow website (or, there was, but disappeared in the first couple of months of 2025). The key quote is:
Conditionally licensed products are required to be pure and safe, and have a reasonable expectation of efficacy.
There may be additional unpublished studies used to support the licensing application … I don’t know.
However, based on the published work what has been approved is:
- a proprietary product containing inactivated Paenibacillus larvae {{11}}
- that reduces a ~50% lethal challenge of AFB spores by ~30%
- and that has never been used outside a Petri dish in the laboratory
Clearly that’s some way from an AFB vaccine that’s going to revolutionise bee health and beekeeping (to say nothing of averting the impending beemageddon).
It might, but there’s a long way to go yet … to see just how far let’s briefly return to the villain of the piece, Paenibacillus larvae, and consider its life cycle.
The biology of Paenibacillus larvae
The only known host of Paenibacillus larvae is the honey bee (see Ebeling et al., 2016). Larvae are infected by ingesting spores of P. larvae supplied in brood food from workers in the colony. Exposure and infection during the first 36 hours after hatching is inevitably fatal.
Spores germinate in the midgut lumen, the bacteria proliferate massively, breach the midgut epithelium and reach the haemocoel. By now the larvae are dead. However, the bacteria continue to proliferate, literally ’turning the larval biomass into bacterial biomass’ (a quote from Ebeling et al., 2016).
By this time the bacteria are starting to starve and so sporulate, eventually drying down in the cell to form a scale containing millions of infectious spores.
How infectious?
Very.
The dose-mortality relationship depends upon the age of the larvae. The standard way to express infectiousness is the dose required to infect or kill 50% of the test subjects, abbreviated to ID50 or LD50 respectively {{12}}. Studies dating back to the 1960’s reported that the LD50 for 0-6 hour larvae was ~200 spores and for 18-24 hour larvae was ~2000 spores (Hoage and Rothenbuhler, 1966).
However, supplementary information in the Hernandez-Lopez paper reports an LD50 in first instar larvae (<24 hours) of only ~20 spores, and this was the dose used as a challenge in their study.
Whether the LD50 is 20 or 200 spores is probably academic … a single dried scale contains millions of spores.
Hope or hype?
A bit of both.
Hope because a 30% reduction in larval infection is better than none at all.
Hype because there’s no evidence this provides colony-level protection in field-realistic situations.
The LD50 quantification studies show that very small numbers of spores can result in infection. An LD50 of ~20 spores in naive larvae results in 50% of them becoming infected, but perhaps ingesting just 5 spores could result in 5% of larvae becoming infected. {{13}}.
If the larvae originated from a vaccinated queen the level needed to infect should be higher.
But would it be high enough?
I’ve no idea.
I don’t know how many spores are transmitted when an exposed worker feeds a larva and I’m not sure anyone does.
The scale of the problem
However, I do have an idea of the levels of spores in honey and hive debris – both of which are likely to be related to spore counts carried by nurse bees – from a recent paper (Kusar et al., 2021).
Honey from asymptomatic hives (no overt disease) in an apiary where other hives had AFB contained ~100 spores/g. The honey from symptomatic hives in the apiary contained 104 to 106 spores/g … 100 to 10,000 times as much.
Hive debris was much worse.
In hives with disease the debris contained 109 spores/g (that’s 1 billion for those unfamiliar with scientific notation). Asymptomatic hives averaged ~105 spores/g but covered a very wide range (0 to ~1010 spores/g).
That’s a lot of spores.
How many spores might foragers pick up and potentially transfer when drifting or robbing nearby hives?
Perhaps there are some studies of this … the assays are available and it’s information that is needed to determine whether vaccinating queen bees is likely to be beneficial in preventing the transmission of AFB.
I hope it works. I also hope that the hype helped raise some VC or investment money to fund the expensive and extensive field trials that will be needed to show that it works well enough.
In my view, only then will it qualify as the world’s first honey bee vaccine.
Note to Facebook users/followers
If you are one of the few hundred that rely on Facebook to get announcements of new posts please instead follow me on Instagram or Twitter or even Mastodon (or subscribe for email notifications – right margin). It is likely that the automagic notifications to Facebook will stop in the next few weeks and I don’t use Facebook as I find it a bit overwhelming shambolic 😉
References
Dickel, F., Bos, N.M.P., Hughes, H., Martín-Hernández, R., Higes, M., Kleiser, A., and Freitak, D. (2022) The oral vaccination with Paenibacillus larvae bacterin can decrease susceptibility to American Foulbrood infection in honey bees—A safety and efficacy study. Frontiers in Veterinary Science 9 https://www.frontiersin.org/articles/10.3389/fvets.2022.946237.
Ebeling, J., Knispel, H., Hertlein, G., Fünfhaus, A., and Genersch, E. (2016) Biology of Paenibacillus larvae, a deadly pathogen of honey bee larvae. Appl Microbiol Biotechnol 100: 7387–7395 https://doi.org/10.1007/s00253-016-7716-0.
Freitak, D., Schmidtberg, H., Dickel, F., Lochnit, G., Vogel, H., and Vilcinskas, A. (2014) The maternal transfer of bacteria can mediate trans-generational immune priming in insects. Virulence 5: 547–554 https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4063815/.
Hernández López, J., Schuehly, W., Crailsheim, K., and Riessberger-Gallé, U. (2014) Trans-generational immune priming in honeybees. Proceedings of the Royal Society B: Biological Sciences 281: 20140454 https://royalsocietypublishing.org/doi/10.1098/rspb.2014.0454.
Hoage, T.R., and Rothenbuhler, W.C. (1966) Larval Honey Bee Response to Various Doses of Bacillus larvae Spores1. Journal of Economic Entomology 59: 42–45 https://doi.org/10.1093/jee/59.1.42.
Kušar, D., Papić, B., Zajc, U., Zdovc, I., Golob, M., Žvokelj, L., et al. (2021) Novel TaqMan PCR Assay for the Quantification of Paenibacillus larvae Spores in Bee-Related Samples. Insects 12: 1034 https://www.mdpi.com/2075-4450/12/11/1034.
Ory, F., Duchemin, V., Kilchenmann, V., Charrière, J.-D., Dainat, B., and Dietemann, V. (2022) Lack of evidence for trans-generational immune priming against the honey bee pathogen Melissococcus plutonius. PLOS ONE 17: e0268142 https://journals.plos.org/plosone/article?id=10.1371/journal.pone.0268142.
Sadd, B.M., Kleinlogel, Y., Schmid-Hempel, R., and Schmid-Hempel, P. (2005) Trans-generational immune priming in a social insect. Biology Letters 1: 386–388 https://royalsocietypublishing.org/doi/10.1098/rsbl.2005.0369.
Tidbury, H.J., Pedersen, A.B., and Boots, M. (2010) Within and transgenerational immune priming in an insect to a DNA virus. Proceedings of the Royal Society B: Biological Sciences 278: 871–876 https://royalsocietypublishing.org/doi/10.1098/rspb.2010.1517.
{{1}}: Weather? Shurely shome mishtake. Nope.
{{2}}: They’re not … at least nothing like as much as many other insects … or the vaquita.
{{3}}: See my earlier post on Feral facts and fallacies
{{4}}: Good photos by Caitlin O’Hara in this article.
{{5}}: An unpleasant task, but someone has to.
{{6}}: Might, as I suspect it’s wrong.
{{7}}: Scientists love making up slightly different abbreviations for exactly the same thing.
{{8}}: i.e. containing no spores.
{{9}}: And if anyone knows the origin of this overused trope I’d be interested. It certainly predates all these movies by decades.
{{10}}: Interestingly – and perhaps disappointingly – 25-30% of hives subsequently ‘died’ which they attribute to frequent inspections when harvesting brood.
{{11}}: No mention of the unicorns or unobtanium.
{{12}}: 50% Infectious Dose or Lethal Dose.
{{13}}: More than enough to justify destruction of the colony.

Join the discussion ...