Does DWV replicate in mites?
Buckle up.
After a gentle tale of bad beekeeping last week {{1}} I think it’s time for a bit of science.
Does deformed wing virus replicate in Varroa mites?
Actually, do any pathogenic virus of honey bees replicate in mites?
Does it matter?
Not really … in comparison to the 2.1 billion people who don’t have access to safe drinking water {{2}}, it’s unimportant.
But if you’re interested in how viruses are transmitted between bees, or how the virus population evolves, then knowing whether the virus replicates in Varroa is important.
For example, perhaps replication in Varroa amplifies a particular sub-population of virus that are more virulent, or more transmissable?
Or causes bees to drift more, so spreading the virus more widely in the environment?
Behaviour-altering viruses are interesting. There has been recent a report that IAPV {{3}} changes the social behaviour of honey bees to potentially increase transmission {{4}}.
So, if it does matter …
Why now?
Three things prompted me to write this post now:
- A recent paper on Black Queen Cell Virus replicating in Varroa was briefly discussed on the Bee-L mailing list. This paper repeated many of the errors of assumption in the historical literature on virus replication in Varroa {{5}}.
- The experiments done to address this question over the last couple of decades have been either contradictory or uninformative. Or both. Or just not very good ?
- We have just published a study {{6}} that specifically addresses whether deformed wing virus can replicate in mites. Inevitably {{7}} I think it’s the most complete answer to date but, as will become clearer, it raises more questions than it answers and I know we don’t yet understand the full story.
To understand the problem you need to have a little background on both viruses and Varroa. Our paper is on deformed wing virus (DWV) and I’ll almost exclusively be using this as an example. If I use the word ‘virus’ I mean DWV.
However, the generalities of the shortcomings in some of the historical studies and the way we tackled the problem is relevant to any virus of honey bees. In that regard, when I use the word ‘virus’ it could probably mean any virus of honey bees.
Background to viruses
Viruses are obligate intracellular parasites. They can only replicate inside a living cell. The majority of pathogenic viruses of honey bees have a genome composed of single stranded positive sense RNA.
I did remind you to buckle up? … here we go.
All single strand positive sense RNA viruses share a broadly similar replication strategy.
The virus enters the cell and the genome is translated to make proteins. There are essentially two types of proteins made:
- the structural proteins – these form the new progeny virus particles. They are what package the new virus genomes into particles (thereby protecting it from damage) to infect the next cell, or host.
- the non-structural proteins – these proteins are the ‘motor’ that makes new virus genomes {{8}}. They do not form part of the virus particle.
After translation the non-structural proteins replicate the virus genome. To do this they first make a single stranded negative-sense template from which they eventually copy hundreds or thousands of more positive-strands.
The positive strands (only) associate with the structural proteins to form new virus particles. These leave the cell and go on to infect another cell, or another bee.
It’s as simple as that ?
A few important things to remember:
- negative strands are never present in the virus particle. Since they would be non-infectious the virus has evolved all sorts of elegant ways of excluding them from the particle.
- there are lots of positive strands produced from each negative strand. A ratio of 1000:1 or higher is not unusual {{9}}.
- without the initial production of the non-structural proteins the virus cannot replicate.
Background to Varroa
I’ve discussed Varroa extensively in previous posts. A full description of the life cycle was presented in the post Know your enemy. For lots of juicy background detail please refer back to that post.
As far as this post is concerned the important facts are as follows:
- Varroa feeds on developing honey bee pupae and, during the (misnamed) phoretic stage of the life cycle, on adult bees.
- the mite ingests a rich ‘soup’ of cells and proteins from the bee. For a long time it was thought that the mite fed on haemolymph (effectively bee blood), but some currently favour the evidence that Varroa feeds on the fat body of bees. As far as this post goes, it’s irrelevant whether the mite feeds on haemolymph or the fat body {{10}}.
- when the mite feeds on a virus-infected bee is also ingests the virus.
- when the mite subsequently feeds on a different bee (for example, to initiate another round of incestuous replication) it transmits some of the virus in its saliva to the new bee.
There, that wasn’t so bad was it?
Evidence for virus replication in Varroa
I’m not going to provide an exhaustive review the extensive literature here. It goes back to the mid-90’s and is pretty dull and rather hard going in places.
Essentially the evidence for replication of viruses in mites is the presence of virus negative strands in the mite. Time and again this has been presented as “definitive proof” that the virus replicates in Varroa.
Does that seem reasonable?
You already know enough Varroa and virus biology to see the flaw in this conclusion.
Q. What did that mite last feed on?
A. A virus-infected pupa.
Q. And what was therefore present in abundance in that pupa?
A. Negative strands of the virus genome.
Just because viral negative strands are detectable in the mite does not mean that they were produced in the mite when (if?) the virus replicated.
Perhaps they were simply ingested with the soup of proteins and cells that the mite eats?
This is an error based upon a positive result. There is an assumption that the positive result tells you something … but it doesn’t, or at least it might not.
Evidence against virus replication in Varroa
This one is more subtle and – in contrast to the above – is an error based on a negative result.
It’s always dangerous drawing scientific conclusions from negative results. Perhaps the experiment just didn’t work? Perhaps it wasn’t sensitive enough? This is why lots of controls are needed.
Scientists looked in detail at all of the proteins present in Varroa mites. They used a method called mass spectroscopy that detects tiny fragmented proteins which they identified by comparison with a database of known proteins.
If one of the tiny fragments matches, there’s a reasonable chance that the entire protein was present. If lots of different tiny fragments match one protein there’s compelling evidence that the entire protein was present in the sample {{11}}.
Using mass spectroscopy scientists detected the structural proteins of deformed wing virus. However, using the same methods, they could not detect the non-structural proteins.
Since the non-structural proteins were ‘absent’ the virus could not have been replicating in the mite as they are a prerequisite for replication.
Seems logical, but is again potentially wrong.
Perhaps the tiny protein fragments of the non-structural proteins of DWV are ‘sticky’ and remain associated with cellular fats and membranes {{12}} during the purification process?
What about controls? Did the authors demonstrate they could detect the non-structural proteins of a virus that does replicate in Varroa? Was the sensitivity of their assay sufficient to detect the levels of non-structural proteins expected to be present? Did they provide evidence they could detect other similarly low abundance proteins?
No.
Should honey bee viruses replicate in Varroa?
Viruses are obligate intracellular parasites. They depend upon proteins and processes in the cell for their replication. This often means that viruses are restricted to a very narrow range of hosts. Sometimes viruses will only replicate in certain tissues or cell types of a single host species.
Varroa and honey bees are both Arthropods, but belong to different classes (Arachnida and Insecta respectively) within this phylum. They are therefore rather distantly related.
This does not mean that a virus of honey bees cannot replicate in the mite. There are several human viruses that also replicate in the mosquito (Dengue, Yellow Fever, Zika for example) during transmission. However, it’s an interesting philosophical cul-de-sac which is distracting us from the main event.
Genetically engineered deformed wing virus
Keeping up?
Good.
Now we’re getting to the more exciting stuff {{13}}.
For some time now my lab have been able to genetically engineer deformed wing virus. This means we can make changes to the virus that help us do interesting or informative experiments.
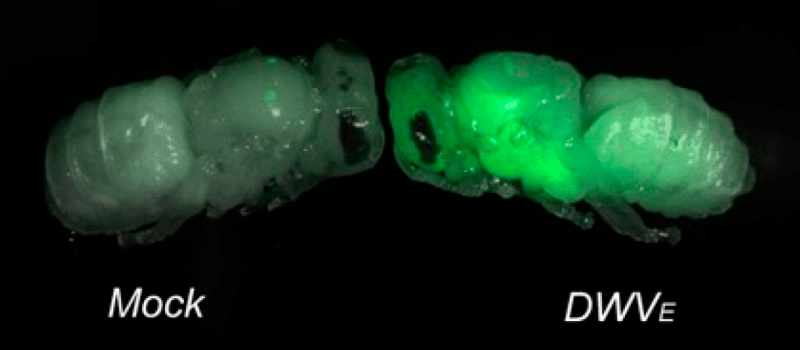
For example, Olesya ‘Alex’ Gusachenko in my team built a virus that expresses a protein that turns bees green.
This is allowing us to look in detail at the specific tissues the virus replicates in, or at transmission of the virus between bees. I’ll discuss green bees again in the future.
To investigate whether deformed wing virus replicates in Varroa we used a virus that had been engineered to contain a tiny genetic tag. It was equivalent to altering 0.05% of the virus genetic material. This genetic modification had three really important features:
- it was unique and was not present in any other strains of deformed wing virus
- although present in the virus genome it did not change any of the characteristics of the virus {{14}}.
- it was easy to detect. Alex developed an assay that could detect really tiny amounts (probably less than 100 copies) of genomes that contained this modification.
An artificial diet for Varroa
The final thing I need to introduce is how to keep Varroa mites in the laboratory {{15}}. The experts can maintain Varroa in incubators, feeding them on little drops of honey bee haemolymph in what are called feed packets.
We’re not experts. But we know people who are.
We collaborated with our friends Alan Bowman, Ewan Campbell and Craig Christie in Aberdeen University who helped us with the Varroa feed packet part of the experiment.
Critically, Alan’s lab have developed a feed packet system that contains no honey bee material. They can maintain Varroa in the lab without feeding them on mushed up bees. This means we had total control over what our Varroa could eat.
The feed packet contents are a closely guarded secret {{16}} but will be published soon.
Enough! Enough! Does DWV replicate in mites?
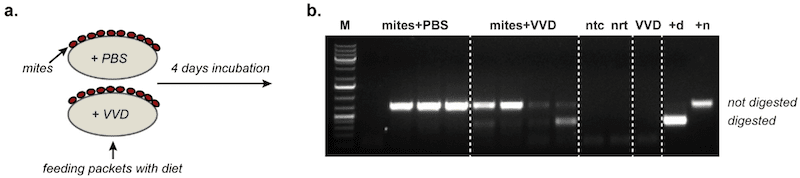
This is how we did the experiment. We grew large amounts of our genetically-tagged virus in honey bee pupae.
We purified the virus and treated it with an enzyme called RNAse.
As described above, the genome of DWV is made from the chemical RNA. RNAse degrades and destroys RNA.
However, viruses are resistant to RNAse because the RNA genome is protected inside the structural protein coat that forms outer layer of the virus particle.
We treated the purified virus preparation with RNAse to remove all RNA that was not inside the virus particles. This contaminating RNA could otherwise compromise the experiment.
Importantly, RNAse degrades both positive and negative strand RNA molecules. Therefore, we could be certain that our virus input into the experiment contained no contaminating negative strand RNA (and confirmed this in several controls).
We then added our virus to the feed packets being used to maintain groups of ten Varroa. After 4 days we harvested the Varroa and extracted all the RNA from them. We also had control mites which were not fed our tagged virus, but were otherwise treated in an identical manner.
All the mites contained very large amount of DWV-specific positive strand RNA. This was unsurprising. Even if our tagged DWV did not replicate in mites they had been feeding on honey bee pupae packed with DWV only four days ago.
We then looked for the presence of negative strand RNA. Again, there were reasonable amounts present, entirely consistent with their previous diet of DWV-infected honey bees. The control mites contained good levels of negative strand RNA.
However, only the mites fed the tagged virus contained a variably weak but consistent signal for the unique genetic tag we had introduced.
We interpret that as very good evidence that DWV does replicate in Varroa.
Is this level of replication significant?
Significant in terms of what?
It’s significant in that this experiment demonstrates for the first time the de novo production of replication intermediates (the negative strand RNA) in mites.
I’m not unbiased ( ? ) but I think this is some of the best evidence supporting DWV replication in Varroa. It also demonstrates the types of experiments that are probably needed to determine whether other honey bee viruses replicate in the mite. The presence of negative strands is not enough. As the figure above shows, at least in the case of DWV, the majority come from the bee pupa the mite last fed on and we have no way to determine whether they are newly replicated or not.
But is the replication significant in terms of the amplification of the virus?
The presence of this signal confirms that there is some replication of DWV in Varroa. The fact that the signal is weak suggests that virus replication is not very extensive or very fast.
For comparison, if we inject the equivalent of 10 DWV virus particles into a honey bee pupa {{17}} they will be replicate to produce 1,000,000,000 (one billion) viruses within 48 hours. Assuming a conservative ratio of positive to negative strands, these individual pupae would each contain a million copies of negative strand RNA two days after infection.
We have yet to fully quantify specific negative strand levels replicated in Varroa, but it looks a whole lot less than that to me.
These are technically very demanding experiments and will take some time to complete. In the meantime we can and should ask a more relevant question.
Is this replication significant in terms of the biology of the virus?
By ‘biology of the virus’ I mean things like differential amplification of specific variants of the virus in the mite, so changing the virus population. For example, does virus adaptation occur, selecting for DWV variants that are more transmissable by Varroa?
We don’t know yet, but at least we have a system which will allow us to test this.
Although we have additional genetically-tagged variants of DWV we can test in the future, in mixed infections one will only be consistently selected over the others if it has a growth advantage.
We don’t yet know such a growth advantage even exists and there might be better ways to determine if it does. These are not straightforward experiments to undertake.
Based upon the limited replication we see in mites I do not think that the replication is significant in terms of increasing the amount of infectious virus in the mite. In contrast, the very high level of replication in honey bees may well allow the evolution of particular strains with particular characteristics.
Where is the replicating virus in Varroa?
We treated the mite like a simple bag of RNA.
We didn’t determine whether the virus was present in muscle, or brain or the ovaries.
The location of the virus is really important.
If the virus is replicating in a tissue that facilitates transmission to the next bee {{18}}, particularly if certain types of virus are being selected because they grow better there, then it might be significant.
Some tissues allow viruses to be transmitted, others do not.
For example, poliovirus is a very distant cousin of DWV. Poliovirus is transmitted faecal-orally {{19}} in contaminated water supplies. After ingestion it replicates really well in the small intestine. It then has a short distance to travel (!) until it is excreted again. So, the primary site of poliovirus replication (the gut), is ideal as it ensures an obvious route for transmission.
Actually, poliovirus replicates so well in the gut that in about 0.5% of infected people it escapes from the gut and enters the peripheral nervous system. It then travels to the spinal cord and finally reaches the brain.
Poliovirus replicates pretty well in the brain as well … so well that it destroys some of the grey matter causing paralytic poliomyelitis {{20}}.
But, as far as the virus is concerned, the brain is a dead-end. There’s no way out. Virus that replicates in the brain can never infect another person. How could it get from the brain to a water supply?
What about DWV? Is DWV replicating in tissues that enables transmission to another host?
Replication in the gut, in salivary glands or possibly the ovaries would all be consistent with transmission (with the ovaries as a source for vertical transmission to progeny mites – though they have already fed at the same spot on the pupa so determining this might be difficult). However, if DWV is replicating in mite muscle or brain it may well be irrelevant in terms of honey bee virus biology as there’s no obvious way out of those tissues.
Questions and answers
We attempted to answer a seemingly simple question “Can DWV replicate in mites?”
We think the answer is yes.
But, as you can see from the few paragraphs above, that short question and even shorter answer has generated lots more questions.
And some of them are much more difficult to answer than the one we tackled ?
Identifying the site(s) of virus replication in Varroa would normally require microdissection of a variety of different tissues. Is the virus present? Is it replicating?
But a virus that expresses a fluorescent green protein during replication should make things a lot more straightforward … but more on that some other time.
Notes
The paper containing this study is available – open access (i.e. free) – the full title and journal details are: Gusachenko et al., (2020) Green Bees: Reverse Genetic Analysis of Deformed Wing Virus Transmission, Replication, and Tropism. Viruses 12: 532.
{{1}}: I can reassure regular readers that there will be plenty more in the future …
{{2}}: But there are things we can do to fix that.
{{3}}: Israeli Acute Paralysis Virus
{{4}}: I’ll discuss this paper in the future …
{{5}}: I’m not going to discuss this again as it’s one of many that reach a conclusion based on the wrong evidence. Have a read yourself after ploughing through my post and see what you think – the paper is Sabahi, Q. et al., (2020). Detection and replication of deformed wing virus and black queen cell virus in parasitic mites, Varroa destructor, from Iranian honey bee (Apis mellifera) colonies. Journal of Apicultural Research, 59: 211-217.
{{7}}: and immodestly.
{{8}}: They do a bunch of other stuff as well … this is the bare-minimum-sketchy-overview version of virus replication.
{{9}}: Some of the first produced positive strands are used to make new negative strand templates, so subsequently leading to an exponential increase in positive strand production.
{{10}}: Chips, mashed, boiled, roasted or dauphinoise … they’re all potatoes.
{{11}}: This is analogous to fingerprint recognition – the more points that are the same, the more certain you are of identifying the individual.
{{12}}: This is not such a daft suggestion. These types of viruses always replicate in association with cell membranes and many of the non-structural proteins have ‘anchors’ that fix them to these membranes.
{{13}}: I need to get out more … like millions under lockdown across the country saying the very same thing.
{{14}}: Think of it like using lowercase or capital letters for the same word. BEE and bee both mean the same thing.
{{15}}: As pets, they’re a big disappointment … don’t bother trying this at home.
{{16}}: I would love to tell you, but then, of course, I’d have to kill you … a quote reportedly from The Hound of the Baskervilles (1901) by Sir Arthur Conan Doyle … though I can’t find it in my copy.
{{17}}: A much smaller amount than we added to the feed packets.
{{18}}: Or the next mite.
{{19}}: Sorry if you’re having your dinner any time soon.
{{20}}: Polio means ‘grey’ in Greek and myelitis means ‘marrow’ … the virus damages the grey marrow of the brain associated with motor function, hence causing paralysis.




Join the discussion ...